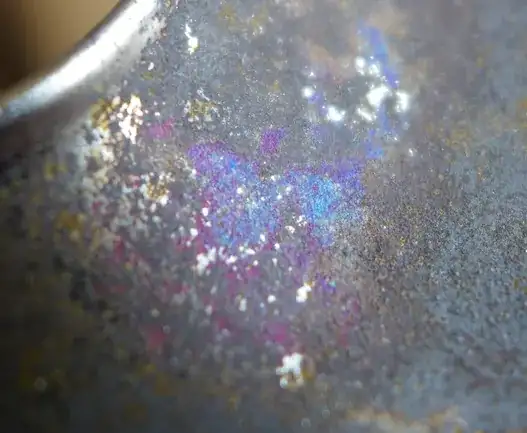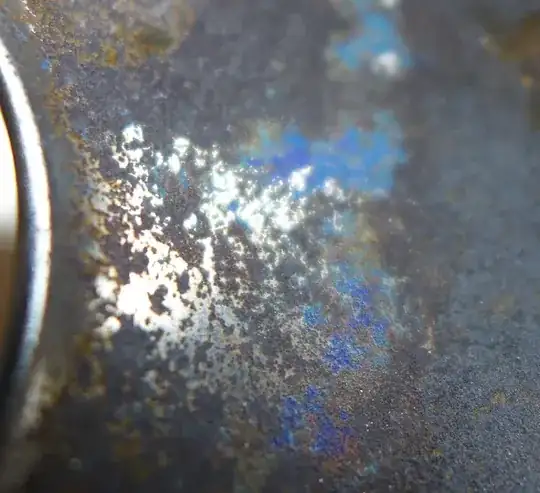Edit3: Bleach will react with copper to produce copper hydroxychloride and as wikipedia says, is commonly found in metal corrosion products. Iron (II) chloride is often greenish colored. Iron and copper chloride will react to produce iron chloride.
Looks like bornite (Cu5FeS4) (yes, bornite is toxic too). But it's unlikley, unless someone spilled some sulfur in the mix. It's definitely a copper mineral of some kind... copper is very commonly used when plating chrome.

Revised answer:
The cups are chrome plated steel. Usually chrome plating starts with copper plated to to steel, then chrome is attached to the copper layer. There may also be other trace metals including nickel, cobalt, tungsten, and zinc.
Why do they plate the cups with chrome? Because chrome plating is shiny (and shiny means it reflects heat better than stainless steel; and chrome is more resistant to corrosion and heat discoloration than steel (actually stainless steel has chrome and other minerals to impart the "stainless" qualities).
Steel will easily discolor (especially stainless steel) when it is heated (like Ecnerwal showed in the picture). However, the discoloration that I see in the picture of your cup is not at all similar to the discoloration of heated steel. The colors of your cup look like distinct "minerals" thact could be scraped off into a bag and analysed by a laboratory.
The brightness of the blue completely suggests a mineral comprised of copper. The location of the colored minerals is another strong indicator that this is a chemical reaction; the minerals were formed in areas of corrosion/oxidation where copper and iron, and possibly nickel and zinc are present. The "peacock" coloration suggests sulfur is/was also present. Sulfur may have come from garlic and onions, or possibly residues from sulfuric acid were present from the plating process, if nickel was used as well.
Edit2 - Other acids will also combine with copper to form other minerals. Many cleaners or "rust emovers" contain phosphoric acid, which could make a form of cornetite (a copper phosphorous mineral) which is darker blue and purple.

Azurite (a copper carbon mineral) is usually more blue than purplish. Malachite (another copper carbon) has just a couple extra % copper than azurite

Cuprite + delefossite (CuFeO2 or Cu1+Fe3+O2) can also yield purples and blues.

So in essence what I'm saying is that this is that copper imparts the blue and or green, and it can combine with iron to make purples, but sulfur really brings out the "peacock" colors. The blue is copper that has oxidized most likely from acidic cleaners.